This is part 4 of a 6 part series
- Detecting Counterfeits Introduction
- Visual Inspection
- Measurements
- Specific Gravity ← you are here
- Currency
- Final Word
Specific gravity is a way to determine the density of an object—or in this case, a coin. The denser an object is, the heavier it feels. In plain language, the density of an object describes how many molecules are packed together in the area. Since gold is one of the densest materials, gold feels heavier than it looks.
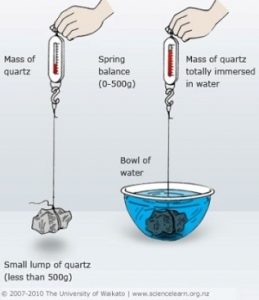
Specific gravity is defined as a ratio of the density of a substance to the density of a standard. In most cases, that standard is purified water. Calculating the specific gravity requires weighing the water and the coin suspended in the water to get the values to complete the calculation. If you are afraid of advanced math or it seems too much work, then you may want to skip this test.To do this test, you need a scale accurate to 1/100th gram, distilled water, a clean container to hold the water, and some way to suspend the coin in the water that does not add to its weight. One way to suspend the coin would be to create a suspension system using sewing thread to make a harness that will hold the coin parallel to the scale, and something to hold up the coin. If you use something other than thread that would add weight and displace more water, you will have to account for the harness. A wooden dowel can be used and laid across two stacks of books or bricks that are of the same height.
First, weigh the coin before doing anything else to get a reference “dry” weight. Then place the coin in the harness, suspend it over the scale, and with the empty container on the scale. Note where the coin sits in the container then remove the coin. Fill the container with distilled water so that it would be about a half-inch higher than the coin then tare the scale—set the weight so that it reads zero.
Now take the suspension system and carefully suspend the coin in the water. When the coin settles in the water, check the side facing down for air bubbles. If there are air bubbles, carefully tilt the coin using the thread to release the bubbles. One way to do this is to grab the threads of the harness with tweezers.
When the coin is still, the weight showing on the scale is weight of the water that was displaced when you suspended the coin in the water. Then you can calculate the specific gravity of the coin by dividing the dry weight by the weight of the displaced water (or dry weight/wet weight). Now you have the specific gravity of the coin.
This calculation may be deceiving because the coin may not be a solid value. An additional calculation would have to be made to account for the other metals in the coin, or you can search the Internet to find tables of the expected specific gravity for the coin you are testing.
Once you have the specific gravity, it should be within .02 percent of the reference value. If it is out of that range, the coin is likely a counterfeit. One way counterfeiters try to fool buyers is to use a lead-based core and plate the coin with the metal. Since lead is lighter than gold and heavier than silver, measuring the specific gravity will tell you if the metal used is the right one.
The following is a good video showing how you can do a basic home test for specific gravity:
Basic Reference
To help you with your tests, the following table will help you with the specific gravity of United States coins:
| Coin Type | Alloy | Specific Gravity |
|---|---|---|
| American Platinum Eagle Bullion | .9995 PI | 21.40 |
| All 24-karat gold coins | .9999 Au | 19.32 |
| American Gold Eagle Bullion | .9167 Au, .30 Ag, .533 Cu | 17.45 |
| Early Gold Eagles | .9167 Au, .0833 Ag+Cu | 17.45 |
| Gold Coins | .900 Au, .100 Cu | 17.16 |
| Classic Head Gold Coins | .8992 Au, .1008 Au | 17.14 |
| American Silver Eagle Bullion | .999 Ag | 10.50 |
| Silver Coins | .900 Ag, .100 Cu | 10.34 |
| Early Silver Coins | .8924 Ag, .1076 Cu | 10.32 |
| Silver Trime | .75 Ag, .25 Cu | 10.11 |
| Silver Clad Halves | .400 Ag, .600 Cu | 9.53 |
| War Nickels | .56 Cu, .35 Ag, .09 Mn | 9.25 |
| Clad Coinage | .75 Cu, .25 Ni | 8.92 |
| Early Copper Coins | 1.00 Cu | 8.92 |
| Early Small Cents | .88 Cu, .12 Ni | 8.92 |
| Bronze Cents | .95 Cu, .05 Zn+Sn | 8.84 |
| Later Bronze Cents | .95 Cu, .05 Zn | 8.84 |
| Modern Small Dollars | .77 Cu, .12 Zn, .07 Mn, .04 Ni | 8.78 |
| Steel Cents | Zinc-plated steel | 7.80 |
| Post 1982 Cents | .975 Zn, .025 Cu | 7.17 |
Chemical symbols used:
- Ag: Silver
- Au: Gold
- Cu: Copper
- Mn: Manganese
- Ni: Nickel
- Pl: Platinum
- Sn: Tin
- Zn: Zinc
In the next installment, we look at the visual inspection of currency.
- Image courtesy of University of Walkato in New Zealand.
- Video courtesy of jdgoony on YouTube.